Ro
-
 KAIST Holds '2025 KAIST Science Frontier Camp' for Multicultural Youth
<2025 KAIST Science Frontier Camp Activities>
KAIST (President Kwang Hyung Lee) announced on the 18th of July that it hosted the '2025 KAIST Science Frontier Camp' for multicultural youth from the 15th for three days and two nights at the Creative Learning Building on its main campus in Daejeon.
This event was organized in accordance with the 'Multicultural Talent Nurturing Agreement' signed by KAIST and GS Caltex in 2024. It marks the first year of a mid-to-long-term project in which 100 million KRW in development funds will be contributed annually for four years. The Global Institute for Talented Education organized the camp, and approximately 30 middle school students from multicultural families affiliated with the 'Hanmaum Educational Volunteer Group' (Director, Honorary Professor Byung Kyu Choi), a mentoring and volunteer organization for multicultural students, participated.
The camp participants enjoyed developing their scientific thinking skills and problem-solving abilities, and broadening their understanding of STEM (Science, Technology, Engineering, and Mathematics) career paths through a variety of science activity programs, including: △'Black Box: Record the Egg's Last Moment!' △'Find the Best Strategy! Heuristic Algorithm Challenge' △'Future Society and AI, Finding Career Directions' △'Distance Dominates the World!' and △'Career Talk Concert.'
During the opening ceremony, Director Byung Kyu Choi delivered a congratulatory speech. Additionally, Yong Hyun Kim, Dean of Admissions at KAIST, gave a special lecture titled 'La La Land KAIST – A Story of Chasing the Dream of a Young Scientist,' sharing honest stories about careers and dreams as a scientist.
Gi Jung Yoo, a freshman from the Division of Undeclared Majors who participated in the camp as a student mentor, shared that he had a very meaningful time mentoring the participating students, who are future STEM hopefuls, sharing vivid experiences as well as insights on metric functions. He added his hope that more students would be given such opportunities.
< Students Actively Taking Part in the Camp Activities>
Si Jong Kwak, Director of the Global Institute for Talented Education, stated, "We hope this will be a practical way to help students foster their interest in science, learn the joy of discussion and communication, and design their future."
KAIST President Kwang Hyung Lee remarked, "This camp was a valuable opportunity for students from diverse cultural backgrounds to gain confidence through science and envision their future." He added, "KAIST will continue to dedicate efforts to nurturing multicultural talent and contribute to creating a sustainable society."
Since 2024, KAIST has introduced and selected multicultural students through its Equal Opportunity Admission track. Utilizing the development funds from GS Caltex, KAIST also established the 'GS Caltex Multicultural Excellence Scholarship Program.' Through this scholarship program, undergraduate students from multicultural families receive living expenses each semester, allowing them to focus more stably on their studies. As the number of applicants for the Equal Opportunity Admission track is increasing every year, more multicultural students are expected to benefit from scholarships in the future.
Additionally, in May, both organizations invited Ms. Si Si Wu Fong, a foreign employee at GS Caltex, to give a special lecture titled 'Working Life for Foreigners in Korea' to support foreign students' career exploration. Foreign students who attended the lecture reported positive feedback, stating that they gained practical career information and were motivated to pursue employment in STEM fields in Korea.
KAIST plans to continue strengthening its efforts to nurture multicultural talent, increase understanding of the upcoming multicultural society, and help spread social values.
<At the 2025 KAIST Science Frontier Camp>
2025.07.18 View 74
KAIST Holds '2025 KAIST Science Frontier Camp' for Multicultural Youth
<2025 KAIST Science Frontier Camp Activities>
KAIST (President Kwang Hyung Lee) announced on the 18th of July that it hosted the '2025 KAIST Science Frontier Camp' for multicultural youth from the 15th for three days and two nights at the Creative Learning Building on its main campus in Daejeon.
This event was organized in accordance with the 'Multicultural Talent Nurturing Agreement' signed by KAIST and GS Caltex in 2024. It marks the first year of a mid-to-long-term project in which 100 million KRW in development funds will be contributed annually for four years. The Global Institute for Talented Education organized the camp, and approximately 30 middle school students from multicultural families affiliated with the 'Hanmaum Educational Volunteer Group' (Director, Honorary Professor Byung Kyu Choi), a mentoring and volunteer organization for multicultural students, participated.
The camp participants enjoyed developing their scientific thinking skills and problem-solving abilities, and broadening their understanding of STEM (Science, Technology, Engineering, and Mathematics) career paths through a variety of science activity programs, including: △'Black Box: Record the Egg's Last Moment!' △'Find the Best Strategy! Heuristic Algorithm Challenge' △'Future Society and AI, Finding Career Directions' △'Distance Dominates the World!' and △'Career Talk Concert.'
During the opening ceremony, Director Byung Kyu Choi delivered a congratulatory speech. Additionally, Yong Hyun Kim, Dean of Admissions at KAIST, gave a special lecture titled 'La La Land KAIST – A Story of Chasing the Dream of a Young Scientist,' sharing honest stories about careers and dreams as a scientist.
Gi Jung Yoo, a freshman from the Division of Undeclared Majors who participated in the camp as a student mentor, shared that he had a very meaningful time mentoring the participating students, who are future STEM hopefuls, sharing vivid experiences as well as insights on metric functions. He added his hope that more students would be given such opportunities.
< Students Actively Taking Part in the Camp Activities>
Si Jong Kwak, Director of the Global Institute for Talented Education, stated, "We hope this will be a practical way to help students foster their interest in science, learn the joy of discussion and communication, and design their future."
KAIST President Kwang Hyung Lee remarked, "This camp was a valuable opportunity for students from diverse cultural backgrounds to gain confidence through science and envision their future." He added, "KAIST will continue to dedicate efforts to nurturing multicultural talent and contribute to creating a sustainable society."
Since 2024, KAIST has introduced and selected multicultural students through its Equal Opportunity Admission track. Utilizing the development funds from GS Caltex, KAIST also established the 'GS Caltex Multicultural Excellence Scholarship Program.' Through this scholarship program, undergraduate students from multicultural families receive living expenses each semester, allowing them to focus more stably on their studies. As the number of applicants for the Equal Opportunity Admission track is increasing every year, more multicultural students are expected to benefit from scholarships in the future.
Additionally, in May, both organizations invited Ms. Si Si Wu Fong, a foreign employee at GS Caltex, to give a special lecture titled 'Working Life for Foreigners in Korea' to support foreign students' career exploration. Foreign students who attended the lecture reported positive feedback, stating that they gained practical career information and were motivated to pursue employment in STEM fields in Korea.
KAIST plans to continue strengthening its efforts to nurture multicultural talent, increase understanding of the upcoming multicultural society, and help spread social values.
<At the 2025 KAIST Science Frontier Camp>
2025.07.18 View 74 -
 KAIST reveals for the first time the mechanism by which alcohol triggers liver inflammation
<(From left)Dr. Keungmo Yang, Professor Won-Il Jeong, Ph.D candidate Kyurae Kim>
Excessive alcohol consumption causes alcoholic liver disease, and about 20% of these cases progress to alcohol-associated steatohepatitis (ASH), which can lead to liver cirrhosis and liver failure. Early diagnosis and treatment are therefore extremely important. A KAIST research team has identified a new molecular mechanism in which alcohol-damaged liver cells increase reactive oxygen species (ROS), leading to cell death and inflammatory responses. In addition, they discovered that Kupffer cells, immune cells residing in the liver, act as a “dual-function regulator” that can either promote or suppress inflammation through interactions with liver cells.
KAIST (President Kwang-Hyung Lee) announced on the 17th that a research team led by Professor Won-Il Jeong from the Graduate School of Medical Science and Engineering, in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center, has uncovered the molecular pathway of liver damage and inflammation caused by alcohol consumption. This finding offers new clues for the diagnosis and treatment of alcohol-associated liver disease (ALD).
Professor Won-Il Jeong’s research team found that during chronic alcohol intake, expression of the vesicular glutamate transporter VGLUT3 increases, leading to glutamate accumulation in hepatocytes. Subsequent binge drinking causes rapid changes in intracellular calcium levels, which then triggers glutamate* secretion. The secreted glutamate stimulates the glutamate receptor mGluR5 on liver-resident macrophages (Kupffer cells), which induces ROS production and activates a pathological pathway resulting in hepatocyte death and inflammation.
*Glutamate: A type of amino acid involved in intercellular signaling, protein synthesis, and energy metabolism in various tissues including the brain and liver. In excess, it can cause overexcitation and death of nerve cells.
<Figure1. Glutamate accumulation in perivenous hepatocytes through vesicular glutamate transporter 3 after 2-week EtOH intake and its release by binge drinking>
A particularly groundbreaking aspect of this study is that damaged hepatocytes and Kupffer cells can form a "pseudosynapse"—a structure similar to a synapse which is previously thought to occur only in the brain—enabling them to exchange signals. This is the first time such a phenomenon has been identified in the liver.
This pseudosynapse forms when hepatocytes expand (ballooning) due to alcohol, becoming physically attached to Kupffer cells. Simply put, the damaged hepatocytes don’t just die—they send distress signals to nearby immune cells, prompting a response.
This discovery proposes a new paradigm: even in peripheral organs, direct structural contact between cells can allow signal transmission. It also shows that damaged hepatocytes can actively stimulate macrophages and induce regeneration through cell death, revealing the liver’s “autonomous recovery function.”
The team also confirmed in animal models that genetic or pharmacological inhibition of VGLUT3, mGluR5, or the ROS-producing enzyme NOX2 reduces alcohol-induced liver damage. They also confirmed that the same mechanism observed in animal models was present in human patients with ALD by analyzing blood and liver tissue samples.
<Figure2. Binge drinking rapidly alters the intracellular calcium levels to release glutamates and activate mGluR5 of Kupffer cells>
Professor Won-Il Jeong of KAIST said, “These findings may serve as new molecular targets for early diagnosis and treatment of ASH in the future.”
This study was jointly led by Dr. Keungmo Yang (now at Yeouido St. Mary’s Hospital) and Kyurae Kim, a doctoral candidate at KAIST, who served as co–first authors. It was conducted in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center and was published in the journal Nature Communications on July 1.
※ Article Title: Binge drinking triggers VGLUT3-mediated glutamate secretion and subsequent hepatic inflammation by activating mGluR5/NOX2 in Kupffer cells ※ DOI: https://doi.org/10.1038/s41467-025-60820-3
This study was supported by the Ministry of Science and ICT through the National Research Foundation of Korea's Global Leader Program, Mid-Career Researcher Program, and the Bio & Medical Technology Development Program.
2025.07.17 View 130
KAIST reveals for the first time the mechanism by which alcohol triggers liver inflammation
<(From left)Dr. Keungmo Yang, Professor Won-Il Jeong, Ph.D candidate Kyurae Kim>
Excessive alcohol consumption causes alcoholic liver disease, and about 20% of these cases progress to alcohol-associated steatohepatitis (ASH), which can lead to liver cirrhosis and liver failure. Early diagnosis and treatment are therefore extremely important. A KAIST research team has identified a new molecular mechanism in which alcohol-damaged liver cells increase reactive oxygen species (ROS), leading to cell death and inflammatory responses. In addition, they discovered that Kupffer cells, immune cells residing in the liver, act as a “dual-function regulator” that can either promote or suppress inflammation through interactions with liver cells.
KAIST (President Kwang-Hyung Lee) announced on the 17th that a research team led by Professor Won-Il Jeong from the Graduate School of Medical Science and Engineering, in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center, has uncovered the molecular pathway of liver damage and inflammation caused by alcohol consumption. This finding offers new clues for the diagnosis and treatment of alcohol-associated liver disease (ALD).
Professor Won-Il Jeong’s research team found that during chronic alcohol intake, expression of the vesicular glutamate transporter VGLUT3 increases, leading to glutamate accumulation in hepatocytes. Subsequent binge drinking causes rapid changes in intracellular calcium levels, which then triggers glutamate* secretion. The secreted glutamate stimulates the glutamate receptor mGluR5 on liver-resident macrophages (Kupffer cells), which induces ROS production and activates a pathological pathway resulting in hepatocyte death and inflammation.
*Glutamate: A type of amino acid involved in intercellular signaling, protein synthesis, and energy metabolism in various tissues including the brain and liver. In excess, it can cause overexcitation and death of nerve cells.
<Figure1. Glutamate accumulation in perivenous hepatocytes through vesicular glutamate transporter 3 after 2-week EtOH intake and its release by binge drinking>
A particularly groundbreaking aspect of this study is that damaged hepatocytes and Kupffer cells can form a "pseudosynapse"—a structure similar to a synapse which is previously thought to occur only in the brain—enabling them to exchange signals. This is the first time such a phenomenon has been identified in the liver.
This pseudosynapse forms when hepatocytes expand (ballooning) due to alcohol, becoming physically attached to Kupffer cells. Simply put, the damaged hepatocytes don’t just die—they send distress signals to nearby immune cells, prompting a response.
This discovery proposes a new paradigm: even in peripheral organs, direct structural contact between cells can allow signal transmission. It also shows that damaged hepatocytes can actively stimulate macrophages and induce regeneration through cell death, revealing the liver’s “autonomous recovery function.”
The team also confirmed in animal models that genetic or pharmacological inhibition of VGLUT3, mGluR5, or the ROS-producing enzyme NOX2 reduces alcohol-induced liver damage. They also confirmed that the same mechanism observed in animal models was present in human patients with ALD by analyzing blood and liver tissue samples.
<Figure2. Binge drinking rapidly alters the intracellular calcium levels to release glutamates and activate mGluR5 of Kupffer cells>
Professor Won-Il Jeong of KAIST said, “These findings may serve as new molecular targets for early diagnosis and treatment of ASH in the future.”
This study was jointly led by Dr. Keungmo Yang (now at Yeouido St. Mary’s Hospital) and Kyurae Kim, a doctoral candidate at KAIST, who served as co–first authors. It was conducted in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center and was published in the journal Nature Communications on July 1.
※ Article Title: Binge drinking triggers VGLUT3-mediated glutamate secretion and subsequent hepatic inflammation by activating mGluR5/NOX2 in Kupffer cells ※ DOI: https://doi.org/10.1038/s41467-025-60820-3
This study was supported by the Ministry of Science and ICT through the National Research Foundation of Korea's Global Leader Program, Mid-Career Researcher Program, and the Bio & Medical Technology Development Program.
2025.07.17 View 130 -
 KAIST Successfully Implements 3D Brain-Mimicking Platform with 6x Higher Precision
<(From left) Dr. Dongjo Yoon, Professor Je-Kyun Park from the Department of Bio and Brain Engineering, (upper right) Professor Yoonkey Nam, Dr. Soo Jee Kim>
Existing three-dimensional (3D) neuronal culture technology has limitations in brain research due to the difficulty of precisely replicating the brain's complex multilayered structure and the lack of a platform that can simultaneously analyze both structure and function. A KAIST research team has successfully developed an integrated platform that can implement brain-like layered neuronal structures using 3D printing technology and precisely measure neuronal activity within them.
KAIST (President Kwang Hyung Lee) announced on the 16th of July that a joint research team led by Professors Je-Kyun Park and Yoonkey Nam from the Department of Bio and Brain Engineering has developed an integrated platform capable of fabricating high-resolution 3D multilayer neuronal networks using low-viscosity natural hydrogels with mechanical properties similar to brain tissue, and simultaneously analyzing their structural and functional connectivity.
Conventional bioprinting technology uses high-viscosity bioinks for structural stability, but this limits neuronal proliferation and neurite growth. Conversely, neural cell-friendly low-viscosity hydrogels are difficult to precisely pattern, leading to a fundamental trade-off between structural stability and biological function. The research team completed a sophisticated and stable brain-mimicking platform by combining three key technologies that enable the precise creation of brain structure with dilute gels, accurate alignment between layers, and simultaneous observation of neuronal activity.
The three core technologies are: ▲ 'Capillary Pinning Effect' technology, which enables the dilute gel (hydrogel) to adhere firmly to a stainless steel mesh (micromesh) to prevent it from flowing, thereby reproducing brain structures with six times greater precision (resolution of 500 μm or less) than conventional methods; ▲ the '3D Printing Aligner,' a cylindrical design that ensures the printed layers are precisely stacked without misalignment, guaranteeing the accurate assembly of multilayer structures and stable integration with microelectrode chips; and ▲ 'Dual-mode Analysis System' technology, which simultaneously measures electrical signals from below and observes cell activity with light (calcium imaging) from above, allowing for the simultaneous verification of the functional operation of interlayer connections through multiple methods.
< Figure 1. Platform integrating brain-structure-mimicking neural network model construction and functional measurement technology>
The research team successfully implemented a three-layered mini-brain structure using 3D printing with a fibrin hydrogel, which has elastic properties similar to those of the brain, and experimentally verified the process of actual neural cells transmitting and receiving signals within it.
Cortical neurons were placed in the upper and lower layers, while the middle layer was left empty but designed to allow neurons to penetrate and connect through it. Electrical signals were measured from the lower layer using a microsensor (electrode chip), and cell activity was observed from the upper layer using light (calcium imaging). The results showed that when electrical stimulation was applied, neural cells in both upper and lower layers responded simultaneously. When a synapse-blocking agent (synaptic blocker) was introduced, the response decreased, proving that the neural cells were genuinely connected and transmitting signals.
Professor Je-Kyun Park of KAIST explained, "This research is a joint development achievement of an integrated platform that can simultaneously reproduce the complex multilayered structure and function of brain tissue. Compared to existing technologies where signal measurement was impossible for more than 14 days, this platform maintains a stable microelectrode chip interface for over 27 days, allowing the real-time analysis of structure-function relationships. It can be utilized in various brain research fields such as neurological disease modeling, brain function research, neurotoxicity assessment, and neuroprotective drug screening in the future."
<Figure 2. Integration process of stacked bioprinting technology and microelectrode chip>
The research, in which Dr. Soo Jee Kim and Dr. Dongjo Yoon from KAIST's Department of Bio and Brain Engineering participated as co-first authors, was published online in the international journal 'Biosensors and Bioelectronics' on June 11, 2025.
※Paper: Hybrid biofabrication of multilayered 3D neuronal networks with structural and functional interlayer connectivity
※DOI: https://doi.org/10.1016/j.bios.2025.117688
2025.07.16 View 128
KAIST Successfully Implements 3D Brain-Mimicking Platform with 6x Higher Precision
<(From left) Dr. Dongjo Yoon, Professor Je-Kyun Park from the Department of Bio and Brain Engineering, (upper right) Professor Yoonkey Nam, Dr. Soo Jee Kim>
Existing three-dimensional (3D) neuronal culture technology has limitations in brain research due to the difficulty of precisely replicating the brain's complex multilayered structure and the lack of a platform that can simultaneously analyze both structure and function. A KAIST research team has successfully developed an integrated platform that can implement brain-like layered neuronal structures using 3D printing technology and precisely measure neuronal activity within them.
KAIST (President Kwang Hyung Lee) announced on the 16th of July that a joint research team led by Professors Je-Kyun Park and Yoonkey Nam from the Department of Bio and Brain Engineering has developed an integrated platform capable of fabricating high-resolution 3D multilayer neuronal networks using low-viscosity natural hydrogels with mechanical properties similar to brain tissue, and simultaneously analyzing their structural and functional connectivity.
Conventional bioprinting technology uses high-viscosity bioinks for structural stability, but this limits neuronal proliferation and neurite growth. Conversely, neural cell-friendly low-viscosity hydrogels are difficult to precisely pattern, leading to a fundamental trade-off between structural stability and biological function. The research team completed a sophisticated and stable brain-mimicking platform by combining three key technologies that enable the precise creation of brain structure with dilute gels, accurate alignment between layers, and simultaneous observation of neuronal activity.
The three core technologies are: ▲ 'Capillary Pinning Effect' technology, which enables the dilute gel (hydrogel) to adhere firmly to a stainless steel mesh (micromesh) to prevent it from flowing, thereby reproducing brain structures with six times greater precision (resolution of 500 μm or less) than conventional methods; ▲ the '3D Printing Aligner,' a cylindrical design that ensures the printed layers are precisely stacked without misalignment, guaranteeing the accurate assembly of multilayer structures and stable integration with microelectrode chips; and ▲ 'Dual-mode Analysis System' technology, which simultaneously measures electrical signals from below and observes cell activity with light (calcium imaging) from above, allowing for the simultaneous verification of the functional operation of interlayer connections through multiple methods.
< Figure 1. Platform integrating brain-structure-mimicking neural network model construction and functional measurement technology>
The research team successfully implemented a three-layered mini-brain structure using 3D printing with a fibrin hydrogel, which has elastic properties similar to those of the brain, and experimentally verified the process of actual neural cells transmitting and receiving signals within it.
Cortical neurons were placed in the upper and lower layers, while the middle layer was left empty but designed to allow neurons to penetrate and connect through it. Electrical signals were measured from the lower layer using a microsensor (electrode chip), and cell activity was observed from the upper layer using light (calcium imaging). The results showed that when electrical stimulation was applied, neural cells in both upper and lower layers responded simultaneously. When a synapse-blocking agent (synaptic blocker) was introduced, the response decreased, proving that the neural cells were genuinely connected and transmitting signals.
Professor Je-Kyun Park of KAIST explained, "This research is a joint development achievement of an integrated platform that can simultaneously reproduce the complex multilayered structure and function of brain tissue. Compared to existing technologies where signal measurement was impossible for more than 14 days, this platform maintains a stable microelectrode chip interface for over 27 days, allowing the real-time analysis of structure-function relationships. It can be utilized in various brain research fields such as neurological disease modeling, brain function research, neurotoxicity assessment, and neuroprotective drug screening in the future."
<Figure 2. Integration process of stacked bioprinting technology and microelectrode chip>
The research, in which Dr. Soo Jee Kim and Dr. Dongjo Yoon from KAIST's Department of Bio and Brain Engineering participated as co-first authors, was published online in the international journal 'Biosensors and Bioelectronics' on June 11, 2025.
※Paper: Hybrid biofabrication of multilayered 3D neuronal networks with structural and functional interlayer connectivity
※DOI: https://doi.org/10.1016/j.bios.2025.117688
2025.07.16 View 128 -
 KAIST Develops Robots That React to Danger Like Humans
<(From left) Ph.D candidate See-On Park, Professor Jongwon Lee, and Professor Shinhyun Choi>
In the midst of the co-development of artificial intelligence and robotic advancements, developing technologies that enable robots to efficiently perceive and respond to their surroundings like humans has become a crucial task. In this context, Korean researchers are gaining attention for newly implementing an artificial sensory nervous system that mimics the sensory nervous system of living organisms without the need for separate complex software or circuitry. This breakthrough technology is expected to be applied in fields such as in ultra-small robots and robotic prosthetics, where intelligent and energy-efficient responses to external stimuli are essential.
KAIST (President Kwang Hyung Lee) announced on July15th that a joint research team led by Endowed Chair Professor Shinhyun Choi of the School of Electrical Engineering at KAIST and Professor Jongwon Lee of the Department of Semiconductor Convergence at Chungnam National University (President Jung Kyum Kim) developed a next-generation neuromorphic semiconductor-based artificial sensory nervous system. This system mimics the functions of a living organism's sensory nervous system, and enables a new type of robotic system that can efficiently responds to external stimuli.
In nature, animals — including humans — ignore safe or familiar stimuli and selectively react sensitively to important or dangerous ones. This selective response helps prevent unnecessary energy consumption while maintaining rapid awareness of critical signals. For instance, the sound of an air conditioner or the feel of clothing against the skin soon become familiar and are disregarded. However, if someone calls your name or a sharp object touches your skin, a rapid focus and response occur. These behaviors are regulated by the 'habituation' and 'sensitization' functions in the sensory nervous system. Attempts have been consistently made to apply these sensory nervous system functions of living organisms in order to create robots that efficiently respond to external environments like humans.
However, implementing complex neural characteristics such as habituation and sensitization in robots has faced difficulties in miniaturization and energy efficiency due to the need for separate software or complex circuitry. In particular, there have been attempts to utilize memristors, a neuromorphic semiconductor. A memristor is a next-generation electrical device, which has been widely utilized as an artificial synapse due to its ability to store analog value in the form of device resistance. However, existing memristors had limitations in mimicking the complex characteristics of the nervous system because they only allowed simple monotonic changes in conductivity.
To overcome these limitations, the research team developed a new memristor capable of reproducing complex neural response patterns such as habituation and sensitization within a single device. By introducing additional layer inside the memristor that alter conductivity in opposite directions, the device can more realistically emulate the dynamic synaptic behaviors of a real nervous system — for example, decreasing its response to repeated safe stimuli but quickly regaining sensitivity when a danger signal is detected.
<New memristor mimicking functions of sensory nervous system such as habituation/sensitization>
Using this new memristor, the research team built an artificial sensory nervous system capable of recognizing touch and pain, an applied it to a robotic hand to test its performance. When safe tactile stimuli were repeatedly applied, the robot hand, which initially reacted sensitively to unfamiliar tactile stimuli, gradually showed habituation characteristics by ignoring the stimuli. Later, when stimuli were applied along with an electric shock, it recognized this as a danger signal and showed sensitization characteristics by reacting sensitively again. Through this, it was experimentally proven that robots can efficiently respond to stimuli like humans without separate complex software or processors, verifying the possibility of developing energy-efficient neuro-inspired robots.
<Robot arm with memristor-based artificial sensory nervous system>
See-On Park, researcher at KAIST, stated, "By mimicking the human sensory nervous system with next-generation semiconductors, we have opened up the possibility of implementing a new concept of robots that are smarter and more energy-efficient in responding to external environments." He added, "This technology is expected to be utilized in various fusion fields of next-generation semiconductors and robotics, such as ultra-small robots, military robots, and medical robots like robotic prosthetics".
This research was published online on July 1st in the international journal 'Nature Communications,' with Ph.D candidate See-On Park as the first author.
Paper Title: Experimental demonstration of third-order memristor-based artificial sensory nervous system for neuro-inspired robotics
DOI: https://doi.org/10.1038/s41467-025-60818-x
This research was supported by the Korea National Research Foundation's Next-Generation Intelligent Semiconductor Technology Development Project, the Mid-Career Researcher Program, the PIM Artificial Intelligence Semiconductor Core Technology Development Project, the Excellent New Researcher Program, and the Nano Convergence Technology Division, National Nanofab Center's (NNFC) Nano-Medical Device Project.
2025.07.16 View 186
KAIST Develops Robots That React to Danger Like Humans
<(From left) Ph.D candidate See-On Park, Professor Jongwon Lee, and Professor Shinhyun Choi>
In the midst of the co-development of artificial intelligence and robotic advancements, developing technologies that enable robots to efficiently perceive and respond to their surroundings like humans has become a crucial task. In this context, Korean researchers are gaining attention for newly implementing an artificial sensory nervous system that mimics the sensory nervous system of living organisms without the need for separate complex software or circuitry. This breakthrough technology is expected to be applied in fields such as in ultra-small robots and robotic prosthetics, where intelligent and energy-efficient responses to external stimuli are essential.
KAIST (President Kwang Hyung Lee) announced on July15th that a joint research team led by Endowed Chair Professor Shinhyun Choi of the School of Electrical Engineering at KAIST and Professor Jongwon Lee of the Department of Semiconductor Convergence at Chungnam National University (President Jung Kyum Kim) developed a next-generation neuromorphic semiconductor-based artificial sensory nervous system. This system mimics the functions of a living organism's sensory nervous system, and enables a new type of robotic system that can efficiently responds to external stimuli.
In nature, animals — including humans — ignore safe or familiar stimuli and selectively react sensitively to important or dangerous ones. This selective response helps prevent unnecessary energy consumption while maintaining rapid awareness of critical signals. For instance, the sound of an air conditioner or the feel of clothing against the skin soon become familiar and are disregarded. However, if someone calls your name or a sharp object touches your skin, a rapid focus and response occur. These behaviors are regulated by the 'habituation' and 'sensitization' functions in the sensory nervous system. Attempts have been consistently made to apply these sensory nervous system functions of living organisms in order to create robots that efficiently respond to external environments like humans.
However, implementing complex neural characteristics such as habituation and sensitization in robots has faced difficulties in miniaturization and energy efficiency due to the need for separate software or complex circuitry. In particular, there have been attempts to utilize memristors, a neuromorphic semiconductor. A memristor is a next-generation electrical device, which has been widely utilized as an artificial synapse due to its ability to store analog value in the form of device resistance. However, existing memristors had limitations in mimicking the complex characteristics of the nervous system because they only allowed simple monotonic changes in conductivity.
To overcome these limitations, the research team developed a new memristor capable of reproducing complex neural response patterns such as habituation and sensitization within a single device. By introducing additional layer inside the memristor that alter conductivity in opposite directions, the device can more realistically emulate the dynamic synaptic behaviors of a real nervous system — for example, decreasing its response to repeated safe stimuli but quickly regaining sensitivity when a danger signal is detected.
<New memristor mimicking functions of sensory nervous system such as habituation/sensitization>
Using this new memristor, the research team built an artificial sensory nervous system capable of recognizing touch and pain, an applied it to a robotic hand to test its performance. When safe tactile stimuli were repeatedly applied, the robot hand, which initially reacted sensitively to unfamiliar tactile stimuli, gradually showed habituation characteristics by ignoring the stimuli. Later, when stimuli were applied along with an electric shock, it recognized this as a danger signal and showed sensitization characteristics by reacting sensitively again. Through this, it was experimentally proven that robots can efficiently respond to stimuli like humans without separate complex software or processors, verifying the possibility of developing energy-efficient neuro-inspired robots.
<Robot arm with memristor-based artificial sensory nervous system>
See-On Park, researcher at KAIST, stated, "By mimicking the human sensory nervous system with next-generation semiconductors, we have opened up the possibility of implementing a new concept of robots that are smarter and more energy-efficient in responding to external environments." He added, "This technology is expected to be utilized in various fusion fields of next-generation semiconductors and robotics, such as ultra-small robots, military robots, and medical robots like robotic prosthetics".
This research was published online on July 1st in the international journal 'Nature Communications,' with Ph.D candidate See-On Park as the first author.
Paper Title: Experimental demonstration of third-order memristor-based artificial sensory nervous system for neuro-inspired robotics
DOI: https://doi.org/10.1038/s41467-025-60818-x
This research was supported by the Korea National Research Foundation's Next-Generation Intelligent Semiconductor Technology Development Project, the Mid-Career Researcher Program, the PIM Artificial Intelligence Semiconductor Core Technology Development Project, the Excellent New Researcher Program, and the Nano Convergence Technology Division, National Nanofab Center's (NNFC) Nano-Medical Device Project.
2025.07.16 View 186 -
 KAIST Ushers in Era of Predicting ‘Optimal Alloys’ Using AI, Without High-Temperature Experiments
<Picture1.(From Left) Prof. Seungbum Hong, Ph.D candidate Youngwoo Choi>
Steel alloys used in automobiles and machinery parts are typically manufactured through a melting process at high temperatures. The phenomenon where the components remain unchanged during melting is called “congruent melting.” KAIST researchers have now addressed this process—traditionally only possible through high-temperature experiments—using artificial intelligence (AI). This study draws attention as it proposes a new direction for future alloy development by predicting in advance how well alloy components will mix during melting, a long-standing challenge in the field.
KAIST (President Kwang Hyung Lee) announced on the 14th of July that Professor Seungbum Hong’s research team from the Department of Materials Science and Engineering, in international collaboration with Professor Chris Wolverton’s group at Northwestern University, has developed a high-accuracy machine learning model that predicts whether alloy components will remain stable during melting. This was achieved using formation energy data derived from Density Functional Theory (DFT)* calculations.
*Density Functional Theory (DFT): A computational quantum mechanical method used to investigate the electronic structure of many-body systems, especially atoms, molecules, and solids, based on electron density.
The research team combined formation energy values obtained via DFT with experimental melting reaction data to train a machine learning model on 4,536 binary compounds.
Among the various machine learning algorithms tested, the XGBoost-based classification model demonstrated the highest accuracy in predicting whether alloys would mix well, achieving a prediction accuracy of approximately 82.5%. The team also applied the Shapley value method* to analyze the key features of the model. One major finding was that sharp changes in the slope of the formation energy curve (referred to as “convex hull sharpness”) were the most significant factor. A steep slope indicates a composition with energetically favorable (i.e., stable) formation.
*Shapley value: An explainability method in AI used to determine how much each feature contributed to a prediction.
The most notable significance of this study is that it predicts alloy melting behavior without performing high-temperature experiments. This is especially useful for materials such as high-entropy alloys or ultra-heat-resistant alloys, which are difficult to handle experimentally. The approach could also be extended to the design of complex multi-component alloy systems in the future.
Furthermore, the physical indicators identified by the AI model showed high consistency with actual experimental results on how well alloys mix and remain stable. This suggests that the model could be broadly applied to the development of various metal materials and the prediction of structural stability.
Professor Seungbum Hong of KAIST stated, “This research demonstrates how data-driven predictive materials development is possible by integrating computational methods, experimental data, and machine learning—departing from the traditional experience-based alloy design.” He added, “In the future, by incorporating state-of-the-art AI techniques such as generative models and reinforcement learning, we could enter an era where completely new alloys are designed automatically.”
<Model performance and feature importance analysis for predicting melting congruency. (a) SHAP summary plot showing the impact of individual features on model predictions. (b) Confusion matrix illustrating the model’s classification performance. (c) Receiver operating characteristic (ROC) curve with an AUC (area under the curve) score of 0.87, indicating a strong classification performance.>
Ph.D. candidate Youngwoo Choi, from the Department of Materials Science and Engineering at KAIST, participated as the first author. The study was published in the May issue of APL Machine Learning, a prestigious journal in the field of machine learning published by the American Institute of Physics, and was selected as a “Featured Article.”
※ Paper title: Machine learning-based melting congruency prediction of binary compounds using density functional theory-calculated formation energy ※ DOI: 10.1063/5.0247514
This research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
2025.07.14 View 99
KAIST Ushers in Era of Predicting ‘Optimal Alloys’ Using AI, Without High-Temperature Experiments
<Picture1.(From Left) Prof. Seungbum Hong, Ph.D candidate Youngwoo Choi>
Steel alloys used in automobiles and machinery parts are typically manufactured through a melting process at high temperatures. The phenomenon where the components remain unchanged during melting is called “congruent melting.” KAIST researchers have now addressed this process—traditionally only possible through high-temperature experiments—using artificial intelligence (AI). This study draws attention as it proposes a new direction for future alloy development by predicting in advance how well alloy components will mix during melting, a long-standing challenge in the field.
KAIST (President Kwang Hyung Lee) announced on the 14th of July that Professor Seungbum Hong’s research team from the Department of Materials Science and Engineering, in international collaboration with Professor Chris Wolverton’s group at Northwestern University, has developed a high-accuracy machine learning model that predicts whether alloy components will remain stable during melting. This was achieved using formation energy data derived from Density Functional Theory (DFT)* calculations.
*Density Functional Theory (DFT): A computational quantum mechanical method used to investigate the electronic structure of many-body systems, especially atoms, molecules, and solids, based on electron density.
The research team combined formation energy values obtained via DFT with experimental melting reaction data to train a machine learning model on 4,536 binary compounds.
Among the various machine learning algorithms tested, the XGBoost-based classification model demonstrated the highest accuracy in predicting whether alloys would mix well, achieving a prediction accuracy of approximately 82.5%. The team also applied the Shapley value method* to analyze the key features of the model. One major finding was that sharp changes in the slope of the formation energy curve (referred to as “convex hull sharpness”) were the most significant factor. A steep slope indicates a composition with energetically favorable (i.e., stable) formation.
*Shapley value: An explainability method in AI used to determine how much each feature contributed to a prediction.
The most notable significance of this study is that it predicts alloy melting behavior without performing high-temperature experiments. This is especially useful for materials such as high-entropy alloys or ultra-heat-resistant alloys, which are difficult to handle experimentally. The approach could also be extended to the design of complex multi-component alloy systems in the future.
Furthermore, the physical indicators identified by the AI model showed high consistency with actual experimental results on how well alloys mix and remain stable. This suggests that the model could be broadly applied to the development of various metal materials and the prediction of structural stability.
Professor Seungbum Hong of KAIST stated, “This research demonstrates how data-driven predictive materials development is possible by integrating computational methods, experimental data, and machine learning—departing from the traditional experience-based alloy design.” He added, “In the future, by incorporating state-of-the-art AI techniques such as generative models and reinforcement learning, we could enter an era where completely new alloys are designed automatically.”
<Model performance and feature importance analysis for predicting melting congruency. (a) SHAP summary plot showing the impact of individual features on model predictions. (b) Confusion matrix illustrating the model’s classification performance. (c) Receiver operating characteristic (ROC) curve with an AUC (area under the curve) score of 0.87, indicating a strong classification performance.>
Ph.D. candidate Youngwoo Choi, from the Department of Materials Science and Engineering at KAIST, participated as the first author. The study was published in the May issue of APL Machine Learning, a prestigious journal in the field of machine learning published by the American Institute of Physics, and was selected as a “Featured Article.”
※ Paper title: Machine learning-based melting congruency prediction of binary compounds using density functional theory-calculated formation energy ※ DOI: 10.1063/5.0247514
This research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
2025.07.14 View 99 -
 KAIST Develops Novel Candidiasis Treatment Overcoming Side Effects and Resistance
<(From left) Ph. D Candidate Ju Yeon Chung, Prof.Hyun Jung Chung, Ph.D candidate Seungju Yang, Ph.D candidate Ayoung Park, Dr. Yoon-Kyoung Hong from Asan Medical Center, Prof. Yong Pil Chong, Dr. Eunhee Jeon>
Candida, a type of fungus, which can spread throughout the body via the bloodstream, leading to organ damage and sepsis. Recently, the incidence of candidiasis has surged due to the increase in immunosuppressive therapies, medical implants, and transplantation. Korean researchers have successfully developed a next-generation treatment that, unlike existing antifungals, selectively acts only on Candida, achieving both high therapeutic efficacy and low side effects simultaneously.
KAIST (President Kwang Hyung Lee) announced on the 8th that a research team led by Professor Hyun-Jung Chung of the Department of Biological Sciences, in collaboration with Professor Yong Pil Jeong's team at Asan Medical Center, developed a gene-based nanotherapy (FTNx) that simultaneously inhibits two key enzymes in the Candida cell wall.
Current antifungal drugs for Candida have low target selectivity, which can affect human cells. Furthermore, their therapeutic efficacy is gradually decreasing due to the emergence of new resistant strains. Especially for immunocompromised patients, the infection progresses rapidly and has a poor prognosis, making the development of new treatments to overcome the limitations of existing therapies urgent.
The developed treatment can be administered systemically, and by combining gene suppression technology with nanomaterial technology, it effectively overcomes the structural limitations of existing compound-based drugs and successfully achieves selective treatment against only Candida.
The research team created a gold nanoparticle-based complex loaded with short DNA fragments called antisense oligonucleotides (ASO), which simultaneously target two crucial enzymes—β-1,3-glucan synthase (FKS1) and chitin synthase (CHS3)—important for forming the cell wall of the Candida fungus.
By applying a surface coating technology that binds to a specific glycolipid structure (a structure combining sugar and fat) on the Candida cell wall, a targeted delivery device was implemented. This successfully achieved a precise targeting effect, ensuring the complex is not delivered to human cells at all but acts selectively only on Candida.
<Figure 1: Overview of antifungal therapy design and experimental approach>
This complex, after entering Candida cells, cleaves the mRNA produced by the FKS1 and CHS3 genes, thereby inhibiting translation and simultaneously blocking the synthesis of cell wall components β-1,3-glucan and chitin. As a result, the
Candida cell wall loses its structural stability and collapses, suppressing bacterial survival and proliferation.
In fact, experiments using a systemic candidiasis model in mice confirmed the therapeutic effect: a significant reduction in
Candida count in the organs, normalization of immune responses, and a notable increase in survival rates were observed in the treated group.
Professor Hyun-Jung Chung, who led the research, stated, "This study presents a method to overcome the issues of human toxicity and drug resistance spread with existing treatments, marking an important turning point by demonstrating the applicability of gene therapy for systemic infections". She added, "We plan to continue research on optimizing administration methods and verifying toxicity for future clinical application."
This research involved Ju Yeon Chung and Yoon-Kyoung Hong as co-first authors , and was published in the international journal 'Nature Communications' on July 1st.
Paper Title: Effective treatment of systemic candidiasis by synergistic targeting of cell wall synthesis
DOI: 10.1038/s41467-025-60684-7
This research was supported by the Ministry of Health and Welfare and the National Research Foundation of Korea.
2025.07.08 View 284
KAIST Develops Novel Candidiasis Treatment Overcoming Side Effects and Resistance
<(From left) Ph. D Candidate Ju Yeon Chung, Prof.Hyun Jung Chung, Ph.D candidate Seungju Yang, Ph.D candidate Ayoung Park, Dr. Yoon-Kyoung Hong from Asan Medical Center, Prof. Yong Pil Chong, Dr. Eunhee Jeon>
Candida, a type of fungus, which can spread throughout the body via the bloodstream, leading to organ damage and sepsis. Recently, the incidence of candidiasis has surged due to the increase in immunosuppressive therapies, medical implants, and transplantation. Korean researchers have successfully developed a next-generation treatment that, unlike existing antifungals, selectively acts only on Candida, achieving both high therapeutic efficacy and low side effects simultaneously.
KAIST (President Kwang Hyung Lee) announced on the 8th that a research team led by Professor Hyun-Jung Chung of the Department of Biological Sciences, in collaboration with Professor Yong Pil Jeong's team at Asan Medical Center, developed a gene-based nanotherapy (FTNx) that simultaneously inhibits two key enzymes in the Candida cell wall.
Current antifungal drugs for Candida have low target selectivity, which can affect human cells. Furthermore, their therapeutic efficacy is gradually decreasing due to the emergence of new resistant strains. Especially for immunocompromised patients, the infection progresses rapidly and has a poor prognosis, making the development of new treatments to overcome the limitations of existing therapies urgent.
The developed treatment can be administered systemically, and by combining gene suppression technology with nanomaterial technology, it effectively overcomes the structural limitations of existing compound-based drugs and successfully achieves selective treatment against only Candida.
The research team created a gold nanoparticle-based complex loaded with short DNA fragments called antisense oligonucleotides (ASO), which simultaneously target two crucial enzymes—β-1,3-glucan synthase (FKS1) and chitin synthase (CHS3)—important for forming the cell wall of the Candida fungus.
By applying a surface coating technology that binds to a specific glycolipid structure (a structure combining sugar and fat) on the Candida cell wall, a targeted delivery device was implemented. This successfully achieved a precise targeting effect, ensuring the complex is not delivered to human cells at all but acts selectively only on Candida.
<Figure 1: Overview of antifungal therapy design and experimental approach>
This complex, after entering Candida cells, cleaves the mRNA produced by the FKS1 and CHS3 genes, thereby inhibiting translation and simultaneously blocking the synthesis of cell wall components β-1,3-glucan and chitin. As a result, the
Candida cell wall loses its structural stability and collapses, suppressing bacterial survival and proliferation.
In fact, experiments using a systemic candidiasis model in mice confirmed the therapeutic effect: a significant reduction in
Candida count in the organs, normalization of immune responses, and a notable increase in survival rates were observed in the treated group.
Professor Hyun-Jung Chung, who led the research, stated, "This study presents a method to overcome the issues of human toxicity and drug resistance spread with existing treatments, marking an important turning point by demonstrating the applicability of gene therapy for systemic infections". She added, "We plan to continue research on optimizing administration methods and verifying toxicity for future clinical application."
This research involved Ju Yeon Chung and Yoon-Kyoung Hong as co-first authors , and was published in the international journal 'Nature Communications' on July 1st.
Paper Title: Effective treatment of systemic candidiasis by synergistic targeting of cell wall synthesis
DOI: 10.1038/s41467-025-60684-7
This research was supported by the Ministry of Health and Welfare and the National Research Foundation of Korea.
2025.07.08 View 284 -
 KAIST Presents a Breakthrough in Overcoming Drug Resistance in Cancer – Hope for Treating Intractable Diseases like Diabetes
<(From the left) Prof. Hyun Uk Kim, Ph.D candiate Hae Deok Jung, Ph.D candidate Jina Lim, Prof.Yoosik Kim from the Department of Chemical and Biomolecular Engineering>
One of the biggest obstacles in cancer treatment is drug resistance in cancer cells. Conventional efforts have focused on identifying new drug targets to eliminate these resistant cells, but such approaches can often lead to even stronger resistance. Now, researchers at KAIST have developed a computational framework to predict key metabolic genes that can re-sensitize resistant cancer cells to treatment. This technique holds promise not only for a variety of cancer therapies but also for treating metabolic diseases such as diabetes.
On the 7th of July, KAIST (President Kwang Hyung Lee) announced that a research team led by Professors Hyun Uk Kim and Yoosik Kim from the Department of Chemical and Biomolecular Engineering had developed a computational framework that predicts metabolic gene targets to re-sensitize drug-resistant breast cancer cells. This was achieved using a metabolic network model capable of simulating human metabolism.
Focusing on metabolic alterations—key characteristics in the formation of drug resistance—the researchers developed a metabolism-based approach to identify gene targets that could enhance drug responsiveness by regulating the metabolism of drug-resistant breast cancer cells.
< Computational framework that can identify metabolic gene targets to revert the metabolic state of the drug-resistant cells to that of the drug-sensitive parental cells>
The team first constructed cell-specific metabolic network models by integrating proteomic data obtained from two different types of drug-resistant MCF7 breast cancer cell lines: one resistant to doxorubicin and the other to paclitaxel. They then performed gene knockout simulations* on all of the metabolic genes and analyzed the results.
*Gene knockout simulation: A computational method to predict changes in a biological network by virtually removing specific genes.
As a result, they discovered that suppressing certain genes could make previously resistant cancer cells responsive to anticancer drugs again. Specifically, they identified GOT1 as a target in doxorubicin-resistant cells, GPI in paclitaxel-resistant cells, and SLC1A5 as a common target for both drugs.
The predictions were experimentally validated by suppressing proteins encoded by these genes, which led to the re-sensitization of the drug-resistant cancer cells.
Furthermore, consistent re-sensitization effects were also observed when the same proteins were inhibited in other types of breast cancer cells that had developed resistance to the same drugs.
Professor Yoosik Kim remarked, “Cellular metabolism plays a crucial role in various intractable diseases including infectious and degenerative conditions. This new technology, which predicts metabolic regulation switches, can serve as a foundational tool not only for treating drug-resistant breast cancer but also for a wide range of diseases that currently lack effective therapies.”
Professor Hyun Uk Kim, who led the study, emphasized, “The significance of this research lies in our ability to accurately predict key metabolic genes that can make resistant cancer cells responsive to treatment again—using only computer simulations and minimal experimental data. This framework can be widely applied to discover new therapeutic targets in various cancers and metabolic diseases.”
The study, in which Ph.D. candidates JinA Lim and Hae Deok Jung from KAIST participated as co-first authors, was published online on June 25 in Proceedings of the National Academy of Sciences (PNAS), a leading multidisciplinary journal that covers top-tier research in life sciences, physics, engineering, and social sciences.
※ Title: Genome-scale knockout simulation and clustering analysis of drug-resistant breast cancer cells reveal drug sensitization targets ※ DOI: https://doi.org/10.1073/pnas.2425384122 ※ Authors: JinA Lim (KAIST, co-first author), Hae Deok Jung (KAIST, co-first author), Han Suk Ryu (Seoul National University Hospital, corresponding author), Yoosik Kim (KAIST, corresponding author), Hyun Uk Kim (KAIST, corresponding author), and five others.
This research was supported by the Ministry of Science and ICT through the National Research Foundation of Korea, and the Electronics and Telecommunications Research Institute (ETRI).
2025.07.08 View 397
KAIST Presents a Breakthrough in Overcoming Drug Resistance in Cancer – Hope for Treating Intractable Diseases like Diabetes
<(From the left) Prof. Hyun Uk Kim, Ph.D candiate Hae Deok Jung, Ph.D candidate Jina Lim, Prof.Yoosik Kim from the Department of Chemical and Biomolecular Engineering>
One of the biggest obstacles in cancer treatment is drug resistance in cancer cells. Conventional efforts have focused on identifying new drug targets to eliminate these resistant cells, but such approaches can often lead to even stronger resistance. Now, researchers at KAIST have developed a computational framework to predict key metabolic genes that can re-sensitize resistant cancer cells to treatment. This technique holds promise not only for a variety of cancer therapies but also for treating metabolic diseases such as diabetes.
On the 7th of July, KAIST (President Kwang Hyung Lee) announced that a research team led by Professors Hyun Uk Kim and Yoosik Kim from the Department of Chemical and Biomolecular Engineering had developed a computational framework that predicts metabolic gene targets to re-sensitize drug-resistant breast cancer cells. This was achieved using a metabolic network model capable of simulating human metabolism.
Focusing on metabolic alterations—key characteristics in the formation of drug resistance—the researchers developed a metabolism-based approach to identify gene targets that could enhance drug responsiveness by regulating the metabolism of drug-resistant breast cancer cells.
< Computational framework that can identify metabolic gene targets to revert the metabolic state of the drug-resistant cells to that of the drug-sensitive parental cells>
The team first constructed cell-specific metabolic network models by integrating proteomic data obtained from two different types of drug-resistant MCF7 breast cancer cell lines: one resistant to doxorubicin and the other to paclitaxel. They then performed gene knockout simulations* on all of the metabolic genes and analyzed the results.
*Gene knockout simulation: A computational method to predict changes in a biological network by virtually removing specific genes.
As a result, they discovered that suppressing certain genes could make previously resistant cancer cells responsive to anticancer drugs again. Specifically, they identified GOT1 as a target in doxorubicin-resistant cells, GPI in paclitaxel-resistant cells, and SLC1A5 as a common target for both drugs.
The predictions were experimentally validated by suppressing proteins encoded by these genes, which led to the re-sensitization of the drug-resistant cancer cells.
Furthermore, consistent re-sensitization effects were also observed when the same proteins were inhibited in other types of breast cancer cells that had developed resistance to the same drugs.
Professor Yoosik Kim remarked, “Cellular metabolism plays a crucial role in various intractable diseases including infectious and degenerative conditions. This new technology, which predicts metabolic regulation switches, can serve as a foundational tool not only for treating drug-resistant breast cancer but also for a wide range of diseases that currently lack effective therapies.”
Professor Hyun Uk Kim, who led the study, emphasized, “The significance of this research lies in our ability to accurately predict key metabolic genes that can make resistant cancer cells responsive to treatment again—using only computer simulations and minimal experimental data. This framework can be widely applied to discover new therapeutic targets in various cancers and metabolic diseases.”
The study, in which Ph.D. candidates JinA Lim and Hae Deok Jung from KAIST participated as co-first authors, was published online on June 25 in Proceedings of the National Academy of Sciences (PNAS), a leading multidisciplinary journal that covers top-tier research in life sciences, physics, engineering, and social sciences.
※ Title: Genome-scale knockout simulation and clustering analysis of drug-resistant breast cancer cells reveal drug sensitization targets ※ DOI: https://doi.org/10.1073/pnas.2425384122 ※ Authors: JinA Lim (KAIST, co-first author), Hae Deok Jung (KAIST, co-first author), Han Suk Ryu (Seoul National University Hospital, corresponding author), Yoosik Kim (KAIST, corresponding author), Hyun Uk Kim (KAIST, corresponding author), and five others.
This research was supported by the Ministry of Science and ICT through the National Research Foundation of Korea, and the Electronics and Telecommunications Research Institute (ETRI).
2025.07.08 View 397 -
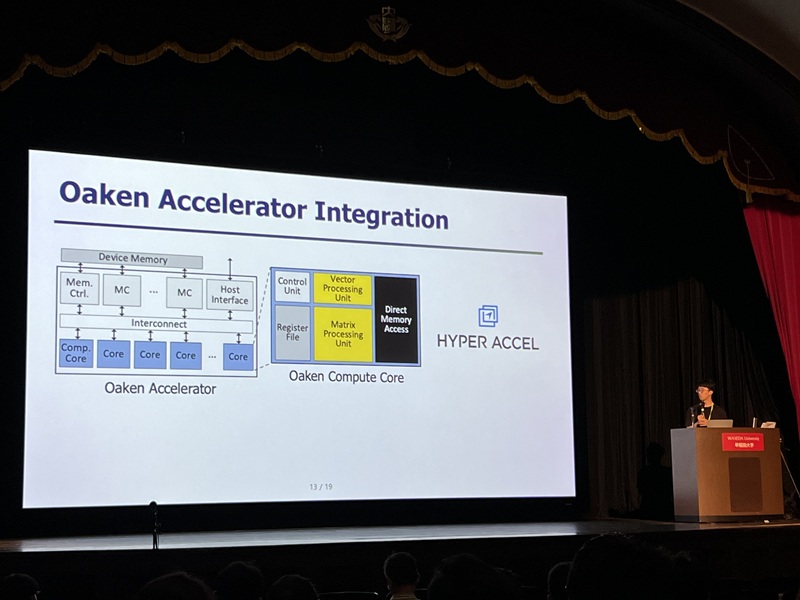 Development of Core NPU Technology to Improve ChatGPT Inference Performance by Over 60%
Latest generative AI models such as OpenAI's ChatGPT-4 and Google's Gemini 2.5 require not only high memory bandwidth but also large memory capacity. This is why generative AI cloud operating companies like Microsoft and Google purchase hundreds of thousands of NVIDIA GPUs. As a solution to address the core challenges of building such high-performance AI infrastructure, Korean researchers have succeeded in developing an NPU (Neural Processing Unit)* core technology that improves the inference performance of generative AI models by an average of over 60% while consuming approximately 44% less power compared to the latest GPUs.
*NPU (Neural Processing Unit): An AI-specific semiconductor chip designed to rapidly process artificial neural networks.
On the 4th, Professor Jongse Park's research team from KAIST School of Computing, in collaboration with HyperAccel Inc. (a startup founded by Professor Joo-Young Kim from the School of Electrical Engineering), announced that they have developed a high-performance, low-power NPU (Neural Processing Unit) core technology specialized for generative AI clouds like ChatGPT.
The technology proposed by the research team has been accepted by the '2025 International Symposium on Computer Architecture (ISCA 2025)', a top-tier international conference in the field of computer architecture.
The key objective of this research is to improve the performance of large-scale generative AI services by lightweighting the inference process, while minimizing accuracy loss and solving memory bottleneck issues. This research is highly recognized for its integrated design of AI semiconductors and AI system software, which are key components of AI infrastructure.
While existing GPU-based AI infrastructure requires multiple GPU devices to meet high bandwidth and capacity demands, this technology enables the configuration of the same level of AI infrastructure using fewer NPU devices through KV cache quantization*. KV cache accounts for most of the memory usage, thereby its quantization significantly reduces the cost of building generative AI clouds.
*KV Cache (Key-Value Cache) Quantization: Refers to reducing the data size in a type of temporary storage space used to improve performance when operating generative AI models (e.g., converting a 16-bit number to a 4-bit number reduces data size by 1/4).
The research team designed it to be integrated with memory interfaces without changing the operational logic of existing NPU architectures. This hardware architecture not only implements the proposed quantization algorithm but also adopts page-level memory management techniques* for efficient utilization of limited memory bandwidth and capacity, and introduces new encoding technique optimized for quantized KV cache.
*Page-level memory management technique: Virtualizes memory addresses, as the CPU does, to allow consistent access within the NPU.
Furthermore, when building an NPU-based AI cloud with superior cost and power efficiency compared to the latest GPUs, the high-performance, low-power nature of NPUs is expected to significantly reduce operating costs.
Professor Jongse Park stated, "This research, through joint work with HyperAccel Inc., found a solution in generative AI inference lightweighting algorithms and succeeded in developing a core NPU technology that can solve the 'memory problem.' Through this technology, we implemented an NPU with over 60% improved performance compared to the latest GPUs by combining quantization techniques that reduce memory requirements while maintaining inference accuracy, and hardware designs optimized for this".
He further emphasized, "This technology has demonstrated the possibility of implementing high-performance, low-power infrastructure specialized for generative AI, and is expected to play a key role not only in AI cloud data centers but also in the AI transformation (AX) environment represented by dynamic, executable AI such as 'Agentic AI'."
This research was presented by Ph.D. student Minsu Kim and Dr. Seongmin Hong from HyperAccel Inc. as co-first authors at the '2025 International Symposium on Computer Architecture (ISCA)' held in Tokyo, Japan, from June 21 to June 25. ISCA, a globally renowned academic conference, received 570 paper submissions this year, with only 127 papers accepted (an acceptance rate of 22.7%).
※Paper Title: Oaken: Fast and Efficient LLM Serving with Online-Offline Hybrid KV Cache Quantization
※DOI: https://doi.org/10.1145/3695053.3731019
Meanwhile, this research was supported by the National Research Foundation of Korea's Excellent Young Researcher Program, the Institute for Information & Communications Technology Planning & Evaluation (IITP), and the AI Semiconductor Graduate School Support Project.
2025.07.07 View 645
Development of Core NPU Technology to Improve ChatGPT Inference Performance by Over 60%
Latest generative AI models such as OpenAI's ChatGPT-4 and Google's Gemini 2.5 require not only high memory bandwidth but also large memory capacity. This is why generative AI cloud operating companies like Microsoft and Google purchase hundreds of thousands of NVIDIA GPUs. As a solution to address the core challenges of building such high-performance AI infrastructure, Korean researchers have succeeded in developing an NPU (Neural Processing Unit)* core technology that improves the inference performance of generative AI models by an average of over 60% while consuming approximately 44% less power compared to the latest GPUs.
*NPU (Neural Processing Unit): An AI-specific semiconductor chip designed to rapidly process artificial neural networks.
On the 4th, Professor Jongse Park's research team from KAIST School of Computing, in collaboration with HyperAccel Inc. (a startup founded by Professor Joo-Young Kim from the School of Electrical Engineering), announced that they have developed a high-performance, low-power NPU (Neural Processing Unit) core technology specialized for generative AI clouds like ChatGPT.
The technology proposed by the research team has been accepted by the '2025 International Symposium on Computer Architecture (ISCA 2025)', a top-tier international conference in the field of computer architecture.
The key objective of this research is to improve the performance of large-scale generative AI services by lightweighting the inference process, while minimizing accuracy loss and solving memory bottleneck issues. This research is highly recognized for its integrated design of AI semiconductors and AI system software, which are key components of AI infrastructure.
While existing GPU-based AI infrastructure requires multiple GPU devices to meet high bandwidth and capacity demands, this technology enables the configuration of the same level of AI infrastructure using fewer NPU devices through KV cache quantization*. KV cache accounts for most of the memory usage, thereby its quantization significantly reduces the cost of building generative AI clouds.
*KV Cache (Key-Value Cache) Quantization: Refers to reducing the data size in a type of temporary storage space used to improve performance when operating generative AI models (e.g., converting a 16-bit number to a 4-bit number reduces data size by 1/4).
The research team designed it to be integrated with memory interfaces without changing the operational logic of existing NPU architectures. This hardware architecture not only implements the proposed quantization algorithm but also adopts page-level memory management techniques* for efficient utilization of limited memory bandwidth and capacity, and introduces new encoding technique optimized for quantized KV cache.
*Page-level memory management technique: Virtualizes memory addresses, as the CPU does, to allow consistent access within the NPU.
Furthermore, when building an NPU-based AI cloud with superior cost and power efficiency compared to the latest GPUs, the high-performance, low-power nature of NPUs is expected to significantly reduce operating costs.
Professor Jongse Park stated, "This research, through joint work with HyperAccel Inc., found a solution in generative AI inference lightweighting algorithms and succeeded in developing a core NPU technology that can solve the 'memory problem.' Through this technology, we implemented an NPU with over 60% improved performance compared to the latest GPUs by combining quantization techniques that reduce memory requirements while maintaining inference accuracy, and hardware designs optimized for this".
He further emphasized, "This technology has demonstrated the possibility of implementing high-performance, low-power infrastructure specialized for generative AI, and is expected to play a key role not only in AI cloud data centers but also in the AI transformation (AX) environment represented by dynamic, executable AI such as 'Agentic AI'."
This research was presented by Ph.D. student Minsu Kim and Dr. Seongmin Hong from HyperAccel Inc. as co-first authors at the '2025 International Symposium on Computer Architecture (ISCA)' held in Tokyo, Japan, from June 21 to June 25. ISCA, a globally renowned academic conference, received 570 paper submissions this year, with only 127 papers accepted (an acceptance rate of 22.7%).
※Paper Title: Oaken: Fast and Efficient LLM Serving with Online-Offline Hybrid KV Cache Quantization
※DOI: https://doi.org/10.1145/3695053.3731019
Meanwhile, this research was supported by the National Research Foundation of Korea's Excellent Young Researcher Program, the Institute for Information & Communications Technology Planning & Evaluation (IITP), and the AI Semiconductor Graduate School Support Project.
2025.07.07 View 645 -
 KAIST Presents Game-Changing Technology for Intractable Brain Disease Treatment Using Micro OLEDs
<(From left)Professor Kyung Cheol Choi, Hyunjoo J. Lee, Somin Lee from the School of Electrical Engineering>
Optogenetics is a technique that controls neural activity by stimulating neurons expressing light-sensitive proteins with specific wavelengths of light. It has opened new possibilities for identifying causes of brain disorders and developing treatments for intractable neurological diseases. Because this technology requires precise stimulation inside the human brain with minimal damage to soft brain tissue, it must be integrated into a neural probe—a medical device implanted in the brain. KAIST researchers have now proposed a new paradigm for neural probes by integrating micro OLEDs into thin, flexible, implantable medical devices.
KAIST (President Kwang Hyung Lee) announced on the 6th of July that Professor Kyung Cheol Choi and researcher Hyunjoo J. Lee from the School of Electrical Engineering have jointly succeeded in developing an optogenetic neural probe integrated with flexible micro OLEDs.
Optical fibers have been used for decades in optogenetic research to deliver light to deep brain regions from external light sources. Recently, research has focused on flexible optical fibers and ultra-miniaturized neural probes that integrate light sources for single-neuron stimulation.
The research team focused on micro OLEDs due to their high spatial resolution and flexibility, which allow for precise light delivery to small areas of neurons. This enables detailed brain circuit analysis while minimizing side effects and avoiding restrictions on animal movement. Moreover, micro OLEDs offer precise control of light wavelengths and support multi-site stimulation, making them suitable for studying complex brain functions.
However, the device's electrical properties degrade easily in the presence of moisture or water, which limited their use as implantable bioelectronics. Furthermore, optimizing the high-resolution integration process on thin, flexible probes remained a challenge.
To address this, the team enhanced the operational reliability of OLEDs in moist, oxygen-rich environments and minimized tissue damage during implantation. They patterned an ultrathin, flexible encapsulation layer* composed of aluminum oxide and parylene-C (Al₂O₃/parylene-C) at widths of 260–600 micrometers (μm) to maintain biocompatibility.
*Encapsulation layer: A barrier that completely blocks oxygen and water molecules from the external environment, ensuring the longevity and reliability of the device.
When integrating the high-resolution micro OLEDs, the researchers also used parylene-C, the same biocompatible material as the encapsulation layer, to maintain flexibility and safety. To eliminate electrical interference between adjacent OLED pixels and spatially separate them, they introduced a pixel define layer (PDL), enabling the independent operation of eight micro OLEDs.
Furthermore, they precisely controlled the residual stress and thickness in the multilayer film structure of the device, ensuring its flexibility even in biological environments. This optimization allowed for probe insertion without bending or external shuttles or needles, minimizing mechanical stress during implantation.
Advanced Functional Materials-Conceptual diagram of a flexible neural probe for integrated optogenetics (Micro-OLED)>
As a result, the team developed a flexible neural probe with integrated micro OLEDs capable of emitting more than one milliwatt per square millimeter (mW/mm²) at 470 nanometers (nm), the optimal wavelength for activating channelrhodopsin-2. This is a significantly high light output for optogenetics and biomedical stimulation applications.
The ultrathin flexible encapsulation layer exhibited a low water vapor transmission rate of 2.66×10⁻⁵ g/m²/day, allowing the device to maintain functionality for over 10 years. The parylene-C-based barrier also demonstrated excellent performance in biological environments, successfully enabling the independent operation of the integrated OLEDs without electrical interference or bending issues.
Dr. Somin Lee, the lead author from Professor Choi’s lab, stated, “We focused on fine-tuning the integration process of highly flexible, high-resolution micro OLEDs onto thin flexible probes, enhancing their biocompatibility and application potential. This is the first reported development of such flexible OLEDs in a probe format and presents a new paradigm for using flexible OLEDs as implantable medical devices for monitoring and therapy.”
This study, with Dr. Somin Lee as the first author, was published online on March 26 in Advanced Functional Materials (IF 18.5), a leading international journal in the field of nanotechnology, and was selected as the cover article for the upcoming July issue.
※ Title: Advanced Micro-OLED Integration on Thin and Flexible Polymer Neural Probes for Targeted Optogenetic Stimulation ※ DOI: https://doi.org/10.1002/adfm.202420758
The research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea through the Electronic Medicine Technology Development Program (Project title: Development of Core Source Technologies and In Vivo Validation for Brain Cognition and Emotion-Enhancing Light-Stimulating Electronic Medicine).
2025.07.07 View 267
KAIST Presents Game-Changing Technology for Intractable Brain Disease Treatment Using Micro OLEDs
<(From left)Professor Kyung Cheol Choi, Hyunjoo J. Lee, Somin Lee from the School of Electrical Engineering>
Optogenetics is a technique that controls neural activity by stimulating neurons expressing light-sensitive proteins with specific wavelengths of light. It has opened new possibilities for identifying causes of brain disorders and developing treatments for intractable neurological diseases. Because this technology requires precise stimulation inside the human brain with minimal damage to soft brain tissue, it must be integrated into a neural probe—a medical device implanted in the brain. KAIST researchers have now proposed a new paradigm for neural probes by integrating micro OLEDs into thin, flexible, implantable medical devices.
KAIST (President Kwang Hyung Lee) announced on the 6th of July that Professor Kyung Cheol Choi and researcher Hyunjoo J. Lee from the School of Electrical Engineering have jointly succeeded in developing an optogenetic neural probe integrated with flexible micro OLEDs.
Optical fibers have been used for decades in optogenetic research to deliver light to deep brain regions from external light sources. Recently, research has focused on flexible optical fibers and ultra-miniaturized neural probes that integrate light sources for single-neuron stimulation.
The research team focused on micro OLEDs due to their high spatial resolution and flexibility, which allow for precise light delivery to small areas of neurons. This enables detailed brain circuit analysis while minimizing side effects and avoiding restrictions on animal movement. Moreover, micro OLEDs offer precise control of light wavelengths and support multi-site stimulation, making them suitable for studying complex brain functions.
However, the device's electrical properties degrade easily in the presence of moisture or water, which limited their use as implantable bioelectronics. Furthermore, optimizing the high-resolution integration process on thin, flexible probes remained a challenge.
To address this, the team enhanced the operational reliability of OLEDs in moist, oxygen-rich environments and minimized tissue damage during implantation. They patterned an ultrathin, flexible encapsulation layer* composed of aluminum oxide and parylene-C (Al₂O₃/parylene-C) at widths of 260–600 micrometers (μm) to maintain biocompatibility.
*Encapsulation layer: A barrier that completely blocks oxygen and water molecules from the external environment, ensuring the longevity and reliability of the device.
When integrating the high-resolution micro OLEDs, the researchers also used parylene-C, the same biocompatible material as the encapsulation layer, to maintain flexibility and safety. To eliminate electrical interference between adjacent OLED pixels and spatially separate them, they introduced a pixel define layer (PDL), enabling the independent operation of eight micro OLEDs.
Furthermore, they precisely controlled the residual stress and thickness in the multilayer film structure of the device, ensuring its flexibility even in biological environments. This optimization allowed for probe insertion without bending or external shuttles or needles, minimizing mechanical stress during implantation.
Advanced Functional Materials-Conceptual diagram of a flexible neural probe for integrated optogenetics (Micro-OLED)>
As a result, the team developed a flexible neural probe with integrated micro OLEDs capable of emitting more than one milliwatt per square millimeter (mW/mm²) at 470 nanometers (nm), the optimal wavelength for activating channelrhodopsin-2. This is a significantly high light output for optogenetics and biomedical stimulation applications.
The ultrathin flexible encapsulation layer exhibited a low water vapor transmission rate of 2.66×10⁻⁵ g/m²/day, allowing the device to maintain functionality for over 10 years. The parylene-C-based barrier also demonstrated excellent performance in biological environments, successfully enabling the independent operation of the integrated OLEDs without electrical interference or bending issues.
Dr. Somin Lee, the lead author from Professor Choi’s lab, stated, “We focused on fine-tuning the integration process of highly flexible, high-resolution micro OLEDs onto thin flexible probes, enhancing their biocompatibility and application potential. This is the first reported development of such flexible OLEDs in a probe format and presents a new paradigm for using flexible OLEDs as implantable medical devices for monitoring and therapy.”
This study, with Dr. Somin Lee as the first author, was published online on March 26 in Advanced Functional Materials (IF 18.5), a leading international journal in the field of nanotechnology, and was selected as the cover article for the upcoming July issue.
※ Title: Advanced Micro-OLED Integration on Thin and Flexible Polymer Neural Probes for Targeted Optogenetic Stimulation ※ DOI: https://doi.org/10.1002/adfm.202420758
The research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea through the Electronic Medicine Technology Development Program (Project title: Development of Core Source Technologies and In Vivo Validation for Brain Cognition and Emotion-Enhancing Light-Stimulating Electronic Medicine).
2025.07.07 View 267 -
 KAIST researcher Se Jin Park develops 'SpeechSSM,' opening up possibilities for a 24-hour AI voice assistant.
<(From Left)Prof. Yong Man Ro and Ph.D. candidate Sejin Park>
Se Jin Park, a researcher from Professor Yong Man Ro’s team at KAIST, has announced 'SpeechSSM', a spoken language model capable of generating long-duration speech that sounds natural and remains consistent.
An efficient processing technique based on linear sequence modeling overcomes the limitations of existing spoken language models, enabling high-quality speech generation without time constraints.
It is expected to be widely used in podcasts, audiobooks, and voice assistants due to its ability to generate natural, long-duration speech like humans.
Recently, Spoken Language Models (SLMs) have been spotlighted as next-generation technology that surpasses the limitations of text-based language models by learning human speech without text to understand and generate linguistic and non-linguistic information. However, existing models showed significant limitations in generating long-duration content required for podcasts, audiobooks, and voice assistants. Now, KAIST researcher has succeeded in overcoming these limitations by developing 'SpeechSSM,' which enables consistent and natural speech generation without time constraints.
KAIST(President Kwang Hyung Lee) announced on the 3rd of July that Ph.D. candidate Sejin Park from Professor Yong Man Ro's research team in the School of Electrical Engineering has developed 'SpeechSSM,' a spoken. a spoken language model capable of generating long-duration speech.
This research is set to be presented as an oral paper at ICML (International Conference on Machine Learning) 2025, one of the top machine learning conferences, selected among approximately 1% of all submitted papers. This not only proves outstanding research ability but also serves as an opportunity to once again demonstrate KAIST's world-leading AI research capabilities.
A major advantage of Spoken Language Models (SLMs) is their ability to directly process speech without intermediate text conversion, leveraging the unique acoustic characteristics of human speakers, allowing for the rapid generation of high-quality speech even in large-scale models.
However, existing models faced difficulties in maintaining semantic and speaker consistency for long-duration speech due to increased 'speech token resolution' and memory consumption when capturing very detailed information by breaking down speech into fine fragments.
To solve this problem, Se Jin Park developed 'SpeechSSM,' a spoken language model using a Hybrid State-Space Model, designed to efficiently process and generate long speech sequences.
This model employs a 'hybrid structure' that alternately places 'attention layers' focusing on recent information and 'recurrent layers' that remember the overall narrative flow (long-term context). This allows the story to flow smoothly without losing coherence even when generating speech for a long time. Furthermore, memory usage and computational load do not increase sharply with input length, enabling stable and efficient learning and the generation of long-duration speech.
SpeechSSM effectively processes unbounded speech sequences by dividing speech data into short, fixed units (windows), processing each unit independently, and then combining them to create long speech.
Additionally, in the speech generation phase, it uses a 'Non-Autoregressive' audio synthesis model (SoundStorm), which rapidly generates multiple parts at once instead of slowly creating one character or one word at a time, enabling the fast generation of high-quality speech.
While existing models typically evaluated short speech models of about 10 seconds, Se Jin Park created new evaluation tasks for speech generation based on their self-built benchmark dataset, 'LibriSpeech-Long,' capable of generating up to 16 minutes of speech.
Compared to PPL (Perplexity), an existing speech model evaluation metric that only indicates grammatical correctness, she proposed new evaluation metrics such as 'SC-L (semantic coherence over time)' to assess content coherence over time, and 'N-MOS-T (naturalness mean opinion score over time)' to evaluate naturalness over time, enabling more effective and precise evaluation.
Through these new evaluations, it was confirmed that speech generated by the SpeechSSM spoken language model consistently featured specific individuals mentioned in the initial prompt, and new characters and events unfolded naturally and contextually consistently, despite long-duration generation. This contrasts sharply with existing models, which tended to easily lose their topic and exhibit repetition during long-duration generation.
PhD candidate Sejin Park explained, "Existing spoken language models had limitations in long-duration generation, so our goal was to develop a spoken language model capable of generating long-duration speech for actual human use." She added, "This research achievement is expected to greatly contribute to various types of voice content creation and voice AI fields like voice assistants, by maintaining consistent content in long contexts and responding more efficiently and quickly in real time than existing methods."
This research, with Se Jin Park as the first author, was conducted in collaboration with Google DeepMind and is scheduled to be presented as an oral presentation at ICML (International Conference on Machine Learning) 2025 on July 16th.
Paper Title: Long-Form Speech Generation with Spoken Language Models
DOI: 10.48550/arXiv.2412.18603
Ph.D. candidate Se Jin Park has demonstrated outstanding research capabilities as a member of Professor Yong Man Ro's MLLM (multimodal large language model) research team, through her work integrating vision, speech, and language. Her achievements include a spotlight paper presentation at 2024 CVPR (Computer Vision and Pattern Recognition) and an Outstanding Paper Award at 2024 ACL (Association for Computational Linguistics).
For more information, you can refer to the publication and accompanying demo: SpeechSSM Publications.
2025.07.04 View 657
KAIST researcher Se Jin Park develops 'SpeechSSM,' opening up possibilities for a 24-hour AI voice assistant.
<(From Left)Prof. Yong Man Ro and Ph.D. candidate Sejin Park>
Se Jin Park, a researcher from Professor Yong Man Ro’s team at KAIST, has announced 'SpeechSSM', a spoken language model capable of generating long-duration speech that sounds natural and remains consistent.
An efficient processing technique based on linear sequence modeling overcomes the limitations of existing spoken language models, enabling high-quality speech generation without time constraints.
It is expected to be widely used in podcasts, audiobooks, and voice assistants due to its ability to generate natural, long-duration speech like humans.
Recently, Spoken Language Models (SLMs) have been spotlighted as next-generation technology that surpasses the limitations of text-based language models by learning human speech without text to understand and generate linguistic and non-linguistic information. However, existing models showed significant limitations in generating long-duration content required for podcasts, audiobooks, and voice assistants. Now, KAIST researcher has succeeded in overcoming these limitations by developing 'SpeechSSM,' which enables consistent and natural speech generation without time constraints.
KAIST(President Kwang Hyung Lee) announced on the 3rd of July that Ph.D. candidate Sejin Park from Professor Yong Man Ro's research team in the School of Electrical Engineering has developed 'SpeechSSM,' a spoken. a spoken language model capable of generating long-duration speech.
This research is set to be presented as an oral paper at ICML (International Conference on Machine Learning) 2025, one of the top machine learning conferences, selected among approximately 1% of all submitted papers. This not only proves outstanding research ability but also serves as an opportunity to once again demonstrate KAIST's world-leading AI research capabilities.
A major advantage of Spoken Language Models (SLMs) is their ability to directly process speech without intermediate text conversion, leveraging the unique acoustic characteristics of human speakers, allowing for the rapid generation of high-quality speech even in large-scale models.
However, existing models faced difficulties in maintaining semantic and speaker consistency for long-duration speech due to increased 'speech token resolution' and memory consumption when capturing very detailed information by breaking down speech into fine fragments.
To solve this problem, Se Jin Park developed 'SpeechSSM,' a spoken language model using a Hybrid State-Space Model, designed to efficiently process and generate long speech sequences.
This model employs a 'hybrid structure' that alternately places 'attention layers' focusing on recent information and 'recurrent layers' that remember the overall narrative flow (long-term context). This allows the story to flow smoothly without losing coherence even when generating speech for a long time. Furthermore, memory usage and computational load do not increase sharply with input length, enabling stable and efficient learning and the generation of long-duration speech.
SpeechSSM effectively processes unbounded speech sequences by dividing speech data into short, fixed units (windows), processing each unit independently, and then combining them to create long speech.
Additionally, in the speech generation phase, it uses a 'Non-Autoregressive' audio synthesis model (SoundStorm), which rapidly generates multiple parts at once instead of slowly creating one character or one word at a time, enabling the fast generation of high-quality speech.
While existing models typically evaluated short speech models of about 10 seconds, Se Jin Park created new evaluation tasks for speech generation based on their self-built benchmark dataset, 'LibriSpeech-Long,' capable of generating up to 16 minutes of speech.
Compared to PPL (Perplexity), an existing speech model evaluation metric that only indicates grammatical correctness, she proposed new evaluation metrics such as 'SC-L (semantic coherence over time)' to assess content coherence over time, and 'N-MOS-T (naturalness mean opinion score over time)' to evaluate naturalness over time, enabling more effective and precise evaluation.
Through these new evaluations, it was confirmed that speech generated by the SpeechSSM spoken language model consistently featured specific individuals mentioned in the initial prompt, and new characters and events unfolded naturally and contextually consistently, despite long-duration generation. This contrasts sharply with existing models, which tended to easily lose their topic and exhibit repetition during long-duration generation.
PhD candidate Sejin Park explained, "Existing spoken language models had limitations in long-duration generation, so our goal was to develop a spoken language model capable of generating long-duration speech for actual human use." She added, "This research achievement is expected to greatly contribute to various types of voice content creation and voice AI fields like voice assistants, by maintaining consistent content in long contexts and responding more efficiently and quickly in real time than existing methods."
This research, with Se Jin Park as the first author, was conducted in collaboration with Google DeepMind and is scheduled to be presented as an oral presentation at ICML (International Conference on Machine Learning) 2025 on July 16th.
Paper Title: Long-Form Speech Generation with Spoken Language Models
DOI: 10.48550/arXiv.2412.18603
Ph.D. candidate Se Jin Park has demonstrated outstanding research capabilities as a member of Professor Yong Man Ro's MLLM (multimodal large language model) research team, through her work integrating vision, speech, and language. Her achievements include a spotlight paper presentation at 2024 CVPR (Computer Vision and Pattern Recognition) and an Outstanding Paper Award at 2024 ACL (Association for Computational Linguistics).
For more information, you can refer to the publication and accompanying demo: SpeechSSM Publications.
2025.07.04 View 657 -
 KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 869
KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 869 -
 PICASSO Technique Drives Biological Molecules into Technicolor
The new imaging approach brings current imaging colors from four to more than 15 for mapping overlapping proteins
Pablo Picasso’s surreal cubist artistic style shifted common features into unrecognizable scenes, but a new imaging approach bearing his namesake may elucidate the most complicated subject: the brain. Employing artificial intelligence to clarify spectral color blending of tiny molecules used to stain specific proteins and other items of research interest, the PICASSO technique, allows researchers to use more than 15 colors to image and parse our overlapping proteins.
The PICASSO developers, based in Korea, published their approach on May 5 in Nature Communications.
Fluorophores — the staining molecules — emit specific colors when excited by a light, but if more than four fluorophores are used, their emitted colors overlap and blend. Researchers previously developed techniques to correct this spectral overlap by precisely defining the matrix of mixed and unmixed images. This measurement depends on reference spectra, found by identifying clear images of only one fluorophore-stained specimen or of multiple, identically prepared specimens that only contain a single fluorophore each.
“Such reference spectra measurement could be complicated to perform in highly heterogeneous specimens, such as the brain, due to the highly varied emission spectra of fluorophores depending on the subregions from which the spectra were measured,” said co-corresponding author Young-Gyu Yoon, professor in the School of Electrical Engineering at KAIST. He explained that the subregions would each need their own spectra reference measurements, making for an inefficient, time-consuming process. “To address this problem, we developed an approach that does not require reference spectra measurements.”
The approach is the “Process of ultra-multiplexed Imaging of biomolecules viA the unmixing of the Signals of Spectrally Overlapping fluorophores,” also known as PICASSO. Ultra-multiplexed imaging refers to visualizing the numerous individual components of a unit. Like a cinema multiplex in which each theater plays a different movie, each protein in a cell has a different role. By staining with fluorophores, researchers can begin to understand those roles.
“We devised a strategy based on information theory; unmixing is performed by iteratively minimizing the mutual information between mixed images,” said co-corresponding author Jae-Byum Chang, professor in the Department of Materials Science and Engineering, KAIST. “This allows us to get away with the assumption that the spatial distribution of different proteins is mutually exclusive and enables accurate information unmixing.”
To demonstrate PICASSO’s capabilities, the researchers applied the technique to imaging a mouse brain. With a single round of staining, they performed 15-color multiplexed imaging of a mouse brain. Although small, mouse brains are still complex, multifaceted organs that can take significant resources to map. According to the researchers, PICASSO can improve the capabilities of other imaging techniques and allow for the use of even more fluorophore colors.
Using one such imaging technique in combination with PICASSO, the team achieved 45-color multiplexed imaging of the mouse brain in only three staining and imaging cycles, according to Yoon.
“PICASSO is a versatile tool for the multiplexed biomolecule imaging of cultured cells, tissue slices and clinical specimens,” Chang said. “We anticipate that PICASSO will be useful for a broad range of applications for which biomolecules’ spatial information is important. One such application the tool would be useful for is revealing the cellular heterogeneities of tumor microenvironments, especially the heterogeneous populations of immune cells, which are closely related to cancer prognoses and the efficacy of cancer therapies.”
The Samsung Research Funding & Incubation Center for Future Technology supported this work. Spectral imaging was performed at the Korea Basic Science Institute Western Seoul Center.
-PublicationJunyoung Seo, Yeonbo Sim, Jeewon Kim, Hyunwoo Kim, In Cho, Hoyeon Nam, Yong-Gyu Yoon, Jae-Byum Chang, “PICASSO allows ultra-multiplexed fluorescence imaging of spatiallyoverlapping proteins without reference spectra measurements,” May 5, Nature Communications (doi.org/10.1038/s41467-022-30168-z)
-ProfileProfessor Jae-Byum ChangDepartment of Materials Science and EngineeringCollege of EngineeringKAIST
Professor Young-Gyu YoonSchool of Electrical EngineeringCollege of EngineeringKAIST
2022.06.22 View 12301
PICASSO Technique Drives Biological Molecules into Technicolor
The new imaging approach brings current imaging colors from four to more than 15 for mapping overlapping proteins
Pablo Picasso’s surreal cubist artistic style shifted common features into unrecognizable scenes, but a new imaging approach bearing his namesake may elucidate the most complicated subject: the brain. Employing artificial intelligence to clarify spectral color blending of tiny molecules used to stain specific proteins and other items of research interest, the PICASSO technique, allows researchers to use more than 15 colors to image and parse our overlapping proteins.
The PICASSO developers, based in Korea, published their approach on May 5 in Nature Communications.
Fluorophores — the staining molecules — emit specific colors when excited by a light, but if more than four fluorophores are used, their emitted colors overlap and blend. Researchers previously developed techniques to correct this spectral overlap by precisely defining the matrix of mixed and unmixed images. This measurement depends on reference spectra, found by identifying clear images of only one fluorophore-stained specimen or of multiple, identically prepared specimens that only contain a single fluorophore each.
“Such reference spectra measurement could be complicated to perform in highly heterogeneous specimens, such as the brain, due to the highly varied emission spectra of fluorophores depending on the subregions from which the spectra were measured,” said co-corresponding author Young-Gyu Yoon, professor in the School of Electrical Engineering at KAIST. He explained that the subregions would each need their own spectra reference measurements, making for an inefficient, time-consuming process. “To address this problem, we developed an approach that does not require reference spectra measurements.”
The approach is the “Process of ultra-multiplexed Imaging of biomolecules viA the unmixing of the Signals of Spectrally Overlapping fluorophores,” also known as PICASSO. Ultra-multiplexed imaging refers to visualizing the numerous individual components of a unit. Like a cinema multiplex in which each theater plays a different movie, each protein in a cell has a different role. By staining with fluorophores, researchers can begin to understand those roles.
“We devised a strategy based on information theory; unmixing is performed by iteratively minimizing the mutual information between mixed images,” said co-corresponding author Jae-Byum Chang, professor in the Department of Materials Science and Engineering, KAIST. “This allows us to get away with the assumption that the spatial distribution of different proteins is mutually exclusive and enables accurate information unmixing.”
To demonstrate PICASSO’s capabilities, the researchers applied the technique to imaging a mouse brain. With a single round of staining, they performed 15-color multiplexed imaging of a mouse brain. Although small, mouse brains are still complex, multifaceted organs that can take significant resources to map. According to the researchers, PICASSO can improve the capabilities of other imaging techniques and allow for the use of even more fluorophore colors.
Using one such imaging technique in combination with PICASSO, the team achieved 45-color multiplexed imaging of the mouse brain in only three staining and imaging cycles, according to Yoon.
“PICASSO is a versatile tool for the multiplexed biomolecule imaging of cultured cells, tissue slices and clinical specimens,” Chang said. “We anticipate that PICASSO will be useful for a broad range of applications for which biomolecules’ spatial information is important. One such application the tool would be useful for is revealing the cellular heterogeneities of tumor microenvironments, especially the heterogeneous populations of immune cells, which are closely related to cancer prognoses and the efficacy of cancer therapies.”
The Samsung Research Funding & Incubation Center for Future Technology supported this work. Spectral imaging was performed at the Korea Basic Science Institute Western Seoul Center.
-PublicationJunyoung Seo, Yeonbo Sim, Jeewon Kim, Hyunwoo Kim, In Cho, Hoyeon Nam, Yong-Gyu Yoon, Jae-Byum Chang, “PICASSO allows ultra-multiplexed fluorescence imaging of spatiallyoverlapping proteins without reference spectra measurements,” May 5, Nature Communications (doi.org/10.1038/s41467-022-30168-z)
-ProfileProfessor Jae-Byum ChangDepartment of Materials Science and EngineeringCollege of EngineeringKAIST
Professor Young-Gyu YoonSchool of Electrical EngineeringCollege of EngineeringKAIST
2022.06.22 View 12301